
K 2SO 4 initially available for reaction: The only extra step in this problem is to convert from milliliters to liters (which I have done in my head). Question: How many grams of BaSO 4 and how many grams of KNO 3 will be produced when 400 mL of 1.8 M BaNO 3 solution reacts with 300 mL of a 1.2 M K 2SO 4 solution? Use the mole ratios from the balanced chemical equation to convert from moles of limiting reactant to moles of product and then use the gram-mole conversion factor from the periodic table to determine the grams of product formed. Once the limiting reactant is determined, the moles of product can be determined. This means that the AgNO 3 willĪll be used up and AgNO 3 is the limiting reactant. That there is not enough AgNO 3 to react with all of the NaCl. There are actually 0.18 moles of AgNO 3 available, which means Finally, determine the moles of product formed (from balanced equation) and grams of product formed (from periodic table gram-mole conversion factor).ĪgNO 3 needed to react with all of the NaCl: Then determine the limiting reactant (using mole ratios from the balanced equation).
First determine the moles of reactants initially present (using the molarity conversion factor). How many grams of AgCl(s) will be produced if 0.2 L of 1.3 M NaCl solution is added to 0.3 L of 0.6 M AgNO 3? Using Molarity to do Limiting Reactant ProblemsĬonsider the following representation of the chemical reaction between NaCl and AgNO 3: How many liters of a 1.2 M HCl solution are needed to provide 0.24 mole HCl?

How many moles of HCl are in 0.5 L of a 1.2 M HCl solution? Model Examples Molarity as a Conversion Factor If the solute is a reactant, these molesĬan be used in limiting reacatant problems to determine theĪmount of product expected from the reaction. (liters) of solution and the molarity is enough to determine
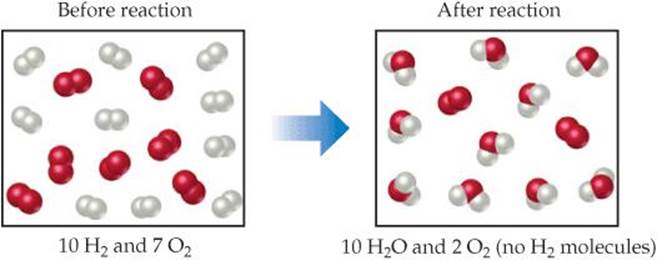
The molarity is a conversion factor between Molarity expresses the amount of solute per liter of solutionĪnd has units of moles of solute per liter of solution (moles The solute and solvent together make the solution. The concentration of the solute in the solvent is an expression of just how much solute is placed into the solvent. Usually there is very little solute and a lot of solvent. Thinking of aqueous solutions, the solute is the material (salt, sugar, etc.) placed into the solvent (water). What is molarity? Molarity is a concentration unit expressed as moles The ideas of balanced chemical reactions, stoichiometry, and limiting reactants can be directly applied to aqueous reactions. Reactions in aqueous (water) solutions are very common and important to understand. Molarity and Limiting Reactants Molarity and Limiting Reactants |


 0 kommentar(er)
0 kommentar(er)
